Sterile Packaging
Reliable Medical Packaging Services
Medical device packaging regulations and requirements are complex and dynamic, and finding reliable sterile packaging services is critical for your company’s supply chain. At Cretex Medical | QTS, we have multiple Class 7 cleanrooms, cross-validated packaging equipment, and cross-trained team members, so that we can easily move staff from one project to another without disrupting any aspect of assembly and packaging for your medical devices.
Package Configuration and Materials
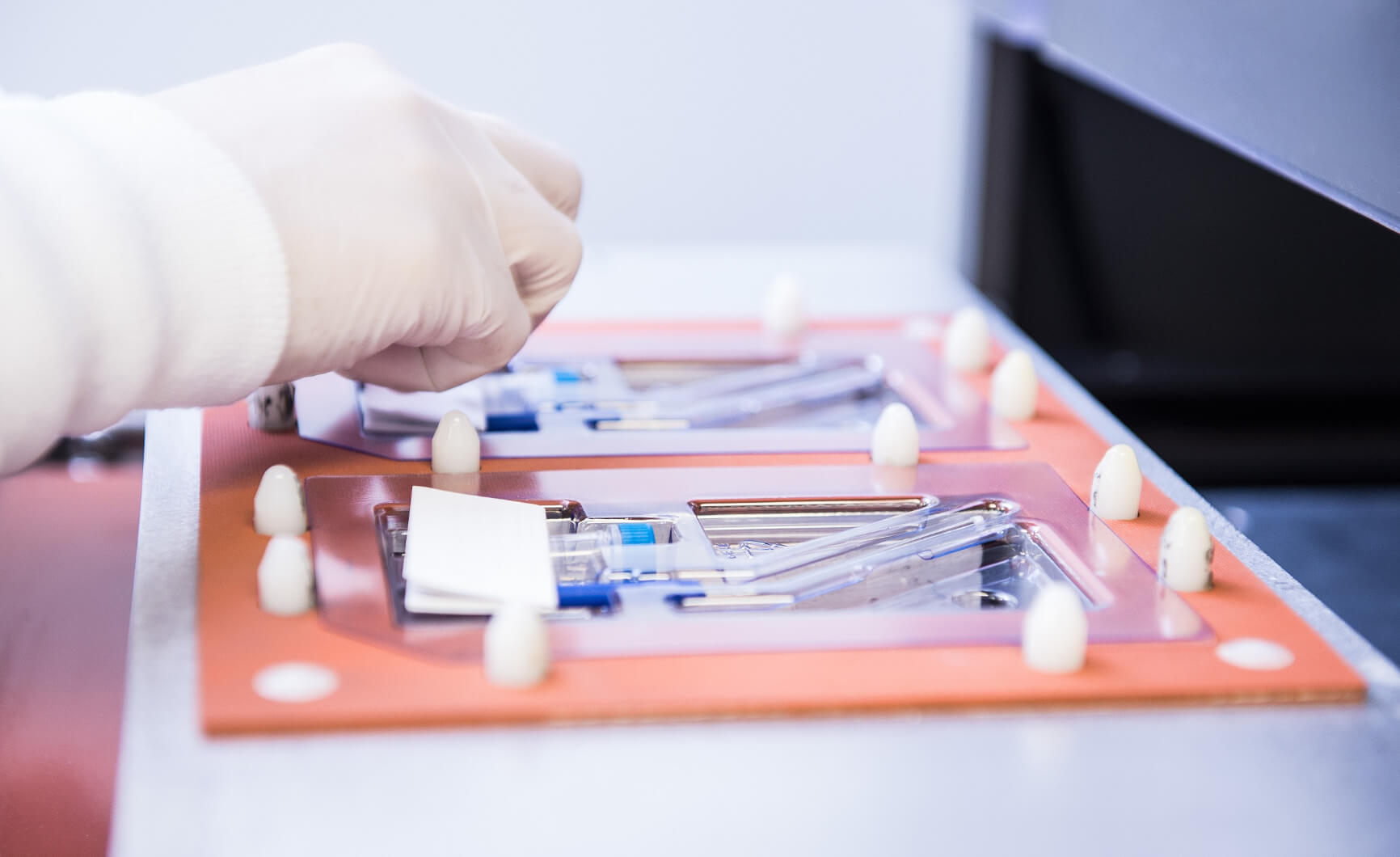
Whether you have your package design complete or are looking for guidance on how to begin, QTS’s team of packaging engineers will collaborate with you to develop a custom package for your device. We have the expertise to help you define your needs and assist you in developing packaging that is effective and appropriate to the distribution and use environments of your products.
Our packaging solutions can incorporate pouches in a variety of materials, single or double barrier trays, or a combination of both including foam, polyurethane or other protective packaging materials to provide the best configuration for your device. QTS also offers QSEAL® Pre-Validated Medical Packaging, an alternative to custom packaging configurations that can help you save money and get your products to market more quickly.
Accelerate your go-to-market process.
Laser Processing, Precision Stamping and Molding Technologies
CDT integrates manufacturing and engineering expertise across a broad range of capabilities to produce high-precision components and assemblies.
Finished Device Assembly, Kitting, Packaging and Sterilization Services
QTS offers reliable end-of-line services including validated cleaning, sterile packaging, and complete kitting and labeling of your devices.
Precision Machining, Additive Manufacturing and Metal Fabrication
rms is a contract manufacturer of high-quality, tight-tolerance implantable devices, components, assemblies, custom instrumentation, metal enclosures, and cases & trays.
